Vegetative propagation
Content:
- Propagation under aseptic conditions (in an in vitro laboratory)
- Meristem culture
- Where to find buds for in vitro propagation
- Node culture
One speaks of vegetative / clonal propagation when the produced plants are identical to the original plant. This type of propagation is always required when you want to make copies of a plant. Some examples from horticultural practice are the propagation of cuttings, the dividing of large plants and air layering. Grafting is also a type of vegetative propagation, which unfortunately cannot be used with orchids.
In the case of orchids, by crossing different parent plants, their properties are combined and the desired plants are created through ongoing selection. If one were to pollinate such a hybrid / cross with its own pollen and cultivate the resulting seeds, then the seedings would no longer have the same properties because the genetic information mixes again during pollination. The only way to multiply a successful cross is through vegetative propagation.
With sympodial growing orchids (e.g. Cattleya, Encyclia, etc.), dividing the plant is the easiest way of vegetative propagation. Monopodial orchids (e.g. Phalaenopsis, Vanda, etc.) are a little more difficult to propagate because there is usually only one shoot without side shoots.
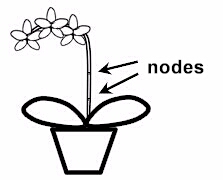
In the genus Phalaenopsis, rooted Keikis, that can be separated and potted, sometimes form at the nodes of the flowering shoot. By applying so-called "keiki pastes" (containing Cytokinins to trigger growth), the dormant buds can be motivated to grow. Some Dendrobium bulbs form new shoots when they are placed on potting material. The flowering shoots of Phaius behave in a very similar way. If you put the flower stalk with the sleeping buds in moss, these buds sprout and form young plants (see Phaius node culture in soil).
Propagation under aseptic conditions (in an in vitro laboratory)
All the methods described so far have the disadvantage that if only a few starting plants are present, only a very small amount of young plants can be produced in a certain time. This is because the young plants cannot be divided often because they need a certain minimum size to be divided and that takes time.
Propagation under aseptic conditions in a laboratory (in vitro propagation) removes this restriction. At the beginning there is always the finding / selection of suitable starting material. In principle, it is possible to regenerate a complete plant from every plant cell. Unfortunately, when only a few cells are used, the risk of mutations is quite high. Since changes in the genome are usually not desired, larger parts of the plant are chosen to start propagation. The first choice here are usually buds, because these have already been created by the plant to form a new shoot and "only" need to be motivated to start growing.

The buds are extracted, cleaned of contamination (fungi & bacteria) and placed on sterile culture media. The media contain all the substances necessary for plant growth. So that the dormant buds starts growing, growth regulators (e.g. Cytokinins) are added to the nutrient medium. Within a few weeks, the buds form one or more young plants, which can then be divided over and over again. Due to the much shorter division interval, this method enables you to produce more plants in the same time.
The number of plants per unit of time can be further increased by not taking the entire dormant bud in culture, but only the tissue in the bud. The following picture shows a maple bud with a red frame marking the tissue to be extracted.

If you cultivate this tissue (cube with a side length of 1-2mm) in a liquid medium with constant movement, it gets the necessary air supply and loses its orientation. The loss of orientation means that the tissue continues to divide and leaves and roots do not develop. As soon as the tissue gets transferred to solid media, leaves and roots form.
Meristem culture
With this type of propagation, only the central, dividing tissue (the meristem) gets extracted from the bud under a microscope. This tissue is the place where new leaves, flowers, etc. emerge (corresponds roughly to a cube with a side length of 0.5 - 1mm).

The advantage of the meristem is that it is usually free of any viruses and bacteria that may be living in the plant and that virus-infected plants can be freed from them (usually in combination with heat treatment). The meristems are cultivated in the same way as the tissue pieces from the buds described in the previous section.
Nurseries often use the word meristem to indicate that the plants offered are copies of a certain mother plant. In most cases, however, the plants were not propagated via the meristem but via the tissue in the bud, which is easier to remove. In both cases, the plants are copies of the original plant.
Where to find buds for in vitro propagation
The easiest way to find these buds is to look at how the plants reproduce vegetatively under normal conditions. As already mentioned in the introduction, some plants (e.g. Phalaenopsis) form Keikis on their flower shoots. This is a sign that there are dormant buds in these places that can be used for propagation. Here are some examples.




The recording of an interesting presentation by Steve Arthur on the subject of "in vitro propagation of Cattleya" is available here. From minute 19 the propagation via buds on new shoots of Cattleya is explained!
Author: Thomas Ederer
